Poor Water Quality May Negatively Impact Pesticide Performance
go.ncsu.edu/readext?901619
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲Water qualities that may impact pesticide application are the water pH, carbonates, water hardness and turbidity. Whether one uses a private well, surface water or a municipal water source, these parameters will vary greatly and should be evaluated before making pesticide applications. Poor water quality can greatly reduce the half-life of pesticides as well as make some product efficacy extremely poor.
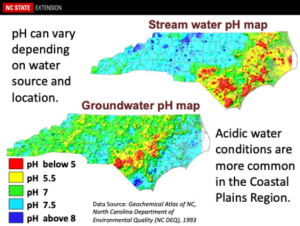
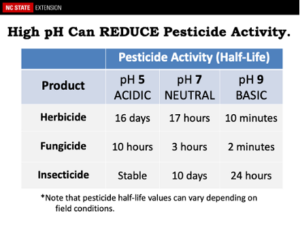
Water pH – Ideally, one should apply pesticides in a water solution that has a pH range of 5.5-6.5. Regrettably, much of the water pH within NC ranges from near neutral to slightly basic. Having thus said, a few shallow wells, especially within the coastal areas of NC, may contain water that are very acidic with water pH of 3 or lower. These water pH values may rapidly reduce the half-life of pesticides, make
Bicarbonate – Bicarbonate, often referred to as alkalinity, is the measure of carbonate (CO3 ) and bicarbonate (HCO3) levels in water. Pesticide water sources with high carbonates may result in a slower absorption rate of the pesticides or result in degradation of a pesticide’s active ingredients to reduce pesticide performance. Furthermore water with high carbonate levels may cause excessive salt accumulation within hoses and nozzles resulting in poor distribution of the pesticides. Pesticides commonly adversely effected by water with high carbonates are usually those that are salt-formulated pesticides such as glyphosate, glufosinate, and 2, 4-D. If the water source is consider as hard water (high sodium and calcium) then even moderate alkalinity increases the probability of poor mixing and rapid degradation of pesticides.
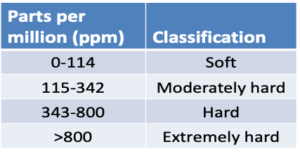
Water Hardness – Water hardness is primarily the measure of calcium and magnesium and is usually expressed as CaC03 ppm. However, other positive ions such as sodium, potassium, iron, or aluminum can interact by binding with some pesticides to form precipitates. These ions bind with active ingredients of pesticides reducing performance by reducing the half-life of pesticides or creating poor mixing/dissolving of product in source water.
Turbidity – Water may contain suspended solids, organic matter, or clay. These particles readily adsorb or bind to some the pesticide’s active ingredients making the product less effective
Typically, larger producers utilize very large tanks to move water from farm to farm. If the pesticide is mixed with this water at the application site and immediately applied, then no negative impacts to pesticide application would be anticipated. However, if pesticides are mixed and stored in a tank with poor water quality, pesticides efficacy may be greatly reduced.
Regrettably, there is no list of pesticides that may be impacted by water quality. Even noting active ingredient of pesticides is not sufficient since the pesticide formulation, additives, or the presence of multiple active ingredients will alter potential reactions. The label of each product must be read to determine what, if any, actions should be taken.
Within North Carolina, the NCDA & CS Agronomic Division Solution Lab offers testing of source water for a small fee. Testing results include water hardness, bicarbonate level (reported as alkalinity within results), water pH, iron content, salt concentration, electrical conductivity, nutrient content and more. Simply fill out Form AD-7 and follow the directions on the form for handling and submission. To collects samples, the water source should be run for at least 10 minutes. Approximately 20 ounces should be collected in a clean plastic bottle. The sample should be refrigerated until sent to the lab.
Corrective actions for poor water will vary. Below are some general guidelines. For more specific discussion of potential corrective measures based on water sample results, contact your local N.C. Cooperative Extension office.
Corrective Measure Based on NCDA & CS Solution Analysis Lab Results
- Add buffering solution to correct for improper solution pH
- Add acid or buffering solution to correct for high alkalinity
- Add ammonium sulfate (AMS)or 30% nitrogen to correct for hard water*
- Mix pesticides in the field and apply within two hours
- Filter turbid water or water with high organic compounds.
- Find an alternative water source if mitigation is not feasible
*Generally, add 8.5 to 17.5 lbs. dry AMS per 100 gallons water or 1.25-2.5% by volume of liquid fertilizer such as 28%N, 32% N or 10-24-0.